Electronic Data Capture
Note
The Electronic Data Capture (EDC) module is available only to users with ROLE_EDC_USER permission. The accessible studies need to be configured to use EDC component.
Concept
This module provides a web user interface to access the EDC system component integrated in RPB platform, see Fig. 8. The main functionality allows browsing studies/sites defined in EDC system and enrolling new subjects using study specific pseudonymisation strategy. It brings the study centric view on subjects enrolled within a projects.

Fig. 8 EDC module left menu items.
Open EDC
Note
ROLE_EDC_OPEN permission is needed to display the link for direct jump into the standalone EDC component.
From portal it is possible to directly navigate to standalone EDC system that is used for the actual eCRF study data collection. These redirection link is visible in top left menu as depicted in Fig. 9.
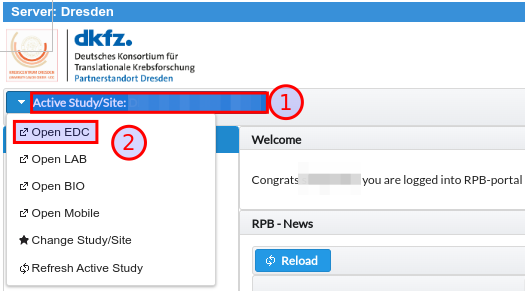
Fig. 9 Open EDC direct link to jump into standalone eCRF collection application.
Subjects/Events/CRFs
Note
ROLE_EDC_USER permission is needed to access this view. It does not currently operate within the scope of currently active study/site, but allows users to quickly switch between project without changing active study.
Partner Sites
This tab presents the project structure of EDC studies defined in RPB platform is limited to two levels, see Fig. 10. The top parent study level holds all subjects enrolled in particular research project, whereas the second site level is limited to patients that e.g. originates for one concrete partner site in the scope of multi-centre trial or are of specific characteristics such as treated tumour entity within the scope of mono-centre trial. Even pure mono-centre trials usually have one site defined that contains all study subjects. The project rights permissions in RPB components allows users to defined access based on these two level hierarchy.
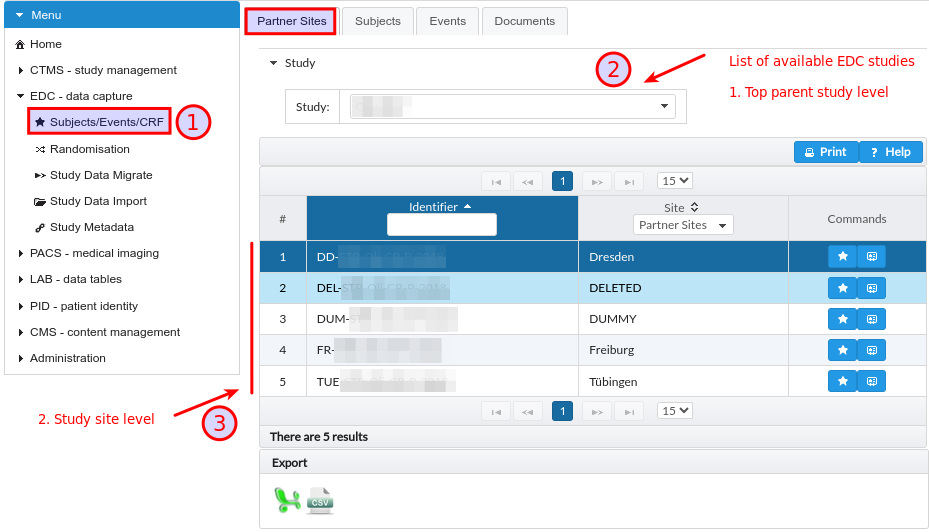
Fig. 10 EDC module study/sites hierarchy.
Note
Additional abstract sites such as DROPOUT, DELETED and DUMMY are often used to control visibility of subjects in the project and logically separate the access to the data.
Subjects
This tab gets loaded when specific parent study and study site was selected in Partner Sites tab and will provide and study subjects enrollment overview, Fig. 13.
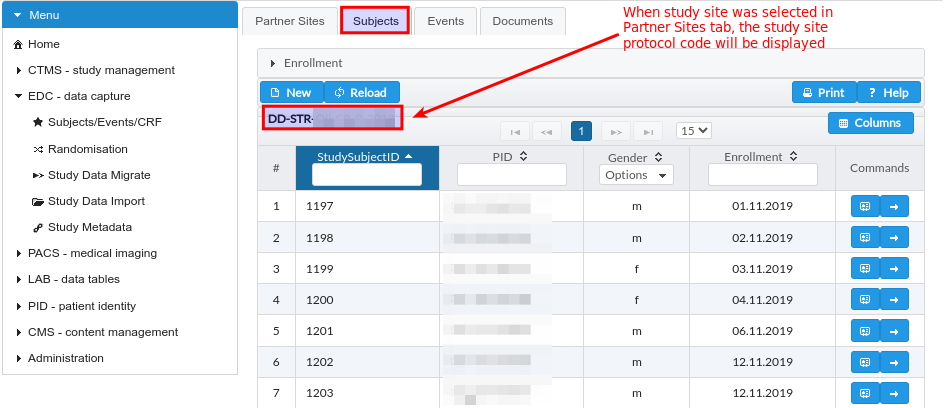
Fig. 13 EDC module study subjects enrollment table.
Events
This tab gets loaded when specific study subject was selected in Subjects tab and will list study events that are available for selected study subject in EDC system in order to allow certain set of administrative operations. Currently this tab view is mostly useful for data managers.
Tasks:
Documents
This tab gets loaded when specific parent study as well as study site was selected in Partner Sites tab and will provide access to documents associated with CTMS record of parent study.
Tasks:
Randomisation
Note
ROLE_EDC_RANDOMISE permission is needed to allow subject randomisation. This view operates within the scope of currently active study/site.
Randomise Study Subjects Visualise Arm Assignments
Study Data Import
Note
ROLE_IMPORT permission is needed to allow bulk import of data into EDC system. This view operates within the scope of currently active study/site.
Bulk Pseudonymisation Bulk Enrollment Bulk Event Schedule Bulk Data Import
Study Metadata
Note
ROLE_EDC_METADATA permission is needed to allow the listing of study metadata items. This view operates within the scope of currently active study/site.
List all data items from eCRFs